Does Hexane Have Hydrogen Bonding
2.12: Intermolecular Forces and Solubilities
- Page ID
- 44653
Learning Objective
- predict whether a mixture of compounds will a class homogeneous or heterogeneous solution
The type of intermolecular forces (IMFs) exhibited past compounds can exist used to predict whether two dissimilar compounds can be mixed to course a homogeneous solution (soluble or miscible). Because organic chemistry can perform reactions in non-aqueous solutions using organic solvents. It is important to consider the solvent as a reaction parameter and the solubility of each reagent. With this said, solvent effects are secondary to the sterics and electrostatics of the reactants. Make sure that you do not drown in the solvent.
Solubility
Virtually all of the organic chemistry that you will run into in this course takes identify in the solution phase. In the organic laboratory, reactions are oft run in nonpolar or slightly polar solvents such as toluene (methylbenzene), hexane, dichloromethane, or diethylether. In recent years, much effort has been fabricated to adjust reaction conditions to let for the use of 'greener' (in other words, more than environmentally friendly) solvents such every bit water or ethanol, which are polar and capable of hydrogen bonding. In organic reactions that occur in the cytosolic region of a cell, the solvent is of grade water. It is disquisitional for any organic chemist to understand the factors which are involved in the solubility of different molecules in dissimilar solvents.
Yous probably remember the rule you learned in general chemistry regarding solubility: 'similar dissolves like' (and even before you lot took any chemistry at all, you probably observed at some point in your life that oil does not mix with water). Let's revisit this erstwhile dominion, and put our knowledge of covalent and noncovalent bonding to work.
Imagine that y'all have a flask filled with h2o, and a selection of substances that you will test to come across how well they dissolve in the water. The first substance is table table salt, or sodium chloride. Equally you would well-nigh certainly predict, specially if you've always inadvertently taken a mouthful of water while swimming in the bounding main, this ionic compound dissolves readily in h2o. Why? Because water, as a very polar molecule, is able to form many ion-dipole interactions with both the sodium cation and the chloride anion, the free energy from which is more than plenty to make up for free energy required to interruption up the ion-ion interactions in the table salt crystal and some water-water hydrogen bonds.
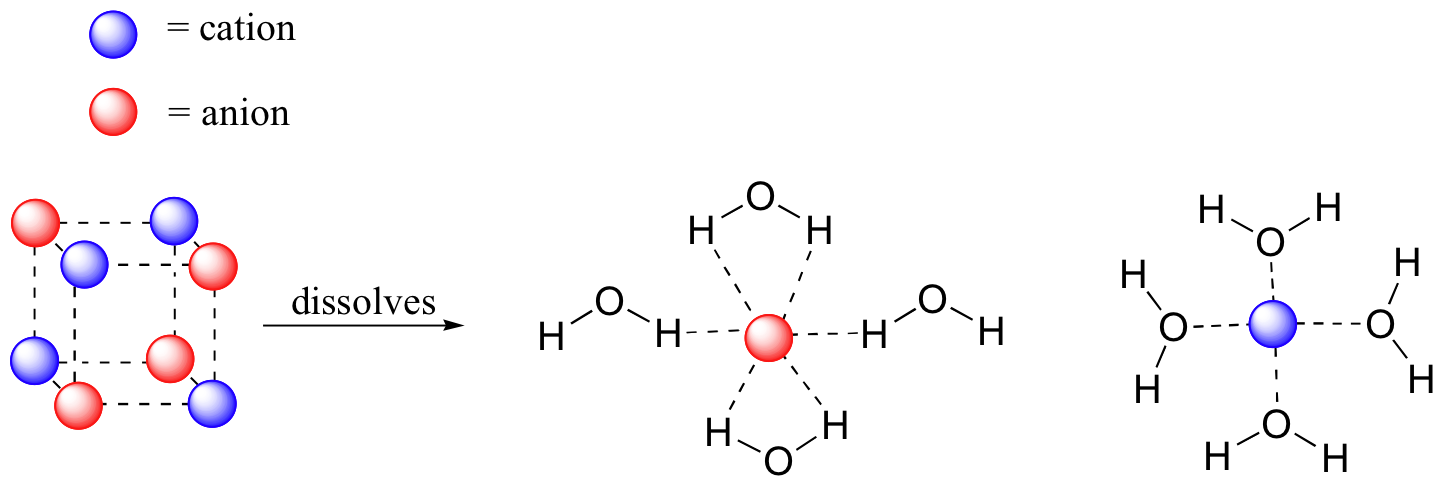
The terminate consequence, then, is that in place of sodium chloride crystals, we have individual sodium cations and chloride anions surrounded past h2o molecules – the salt is now in solution. Charged species as a rule deliquesce readily in h2o: in other words, they are very hydrophilic (h2o-loving).
At present, we'll try a compound chosen biphenyl, which, like sodium chloride, is a colorless crystalline substance (the 2 compounds are readily distinguishable by sight, however – the crystals wait quite unlike).
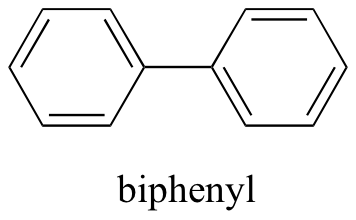
Biphenyl does not dissolve at all in water. Why is this? Because it is a very non-polar molecule, with only carbon-carbon and carbon-hydrogen bonds. Information technology is able to bond to itself very well through nonpolar (London dispersion) interactions, simply information technology is not able to form significant attractive interactions with the very polar solvent molecules. Thus, the energetic cost of breaking up the biphenyl-to-biphenyl interactions in the solid is high, and very little is gained in terms of new biphenyl-water interactions. Water is a terrible solvent for nonpolar hydrocarbon molecules: they are very hydrophobic ('water-fearing').
Next, you endeavor a series of increasingly large alcohol compounds, starting with methanol (i carbon) and catastrophe with octanol (8 carbons).
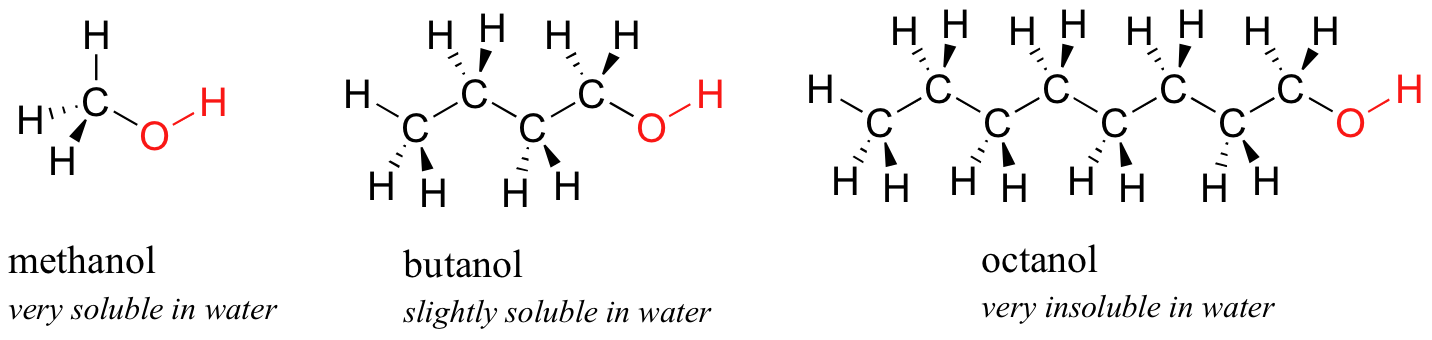
Yous observe that the smaller alcohols - methanol, ethanol, and propanol - dissolve hands in water. This is because the water is able to form hydrogen bonds with the hydroxyl group in these molecules, and the combined energy of germination of these water-alcohol hydrogen bonds is more than than enough to make upwards for the free energy that is lost when the booze-alcohol hydrogen bonds are broken up. When you attempt butanol, yet, you begin to discover that, as you add more and more to the water, it starts to course its ain layer on acme of the h2o.
The longer-chain alcohols - pentanol, hexanol, heptanol, and octanol - are increasingly non-soluble. What is happening here? Clearly, the same favorable water-alcohol hydrogen bonds are still possible with these larger alcohols. The difference, of course, is that the larger alcohols have larger nonpolar, hydrophobic regions in add-on to their hydrophilic hydroxyl group. At nearly iv or five carbons, the hydrophobic result begins to overcome the hydrophilic effect, and water solubility is lost.
At present, endeavor dissolving glucose in the water – even though it has six carbons just like hexanol, information technology besides has 5 hydrogen-bonding, hydrophilic hydroxyl groups in add-on to a sixth oxygen that is capable of being a hydrogen bail acceptor.
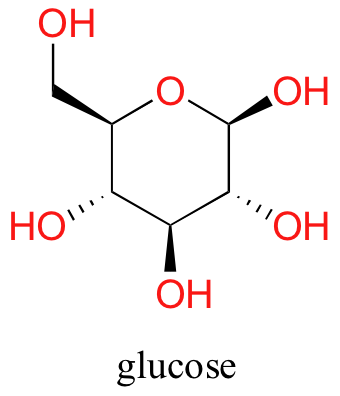
We have tipped the scales to the hydrophilic side, and we detect that glucose is quite soluble in water.
We saw that ethanol was very water-soluble (if it were not, drinking beer or vodka would exist rather inconvenient!) How nigh dimethyl ether, which is a constitutional isomer of ethanol but with an ether rather than an alcohol functional group? We discover that diethyl ether is much less soluble in water. Is information technology capable of forming hydrogen bonds with water? Yeah, in fact, it is –the ether oxygen tin human activity as a hydrogen-bail acceptor. The departure between the ether group and the booze group, however, is that the alcohol group is both a hydrogen bail donor and acceptor.
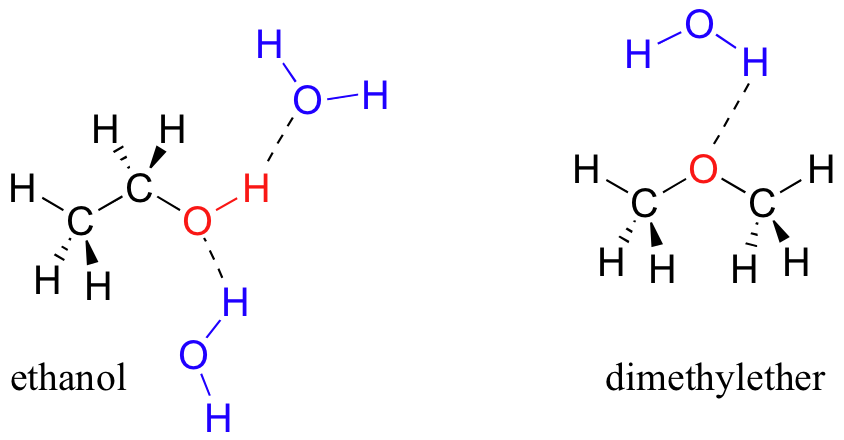
The upshot is that the booze is able to form more energetically favorable interactions with the solvent compared to the ether, and the alcohol is therefore more than soluble.
Here is some other like shooting fish in a barrel experiment that can be done (with proper supervision) in an organic laboratory. Try dissolving benzoic acrid crystals in room temperature water – yous'll find that it is not soluble. Every bit nosotros will learn when nosotros study acid-base chemical science in a after chapter, carboxylic acids such every bit benzoic acid are relatively weak acids, and thus exist by and large in the acidic (protonated) grade when added to pure water.
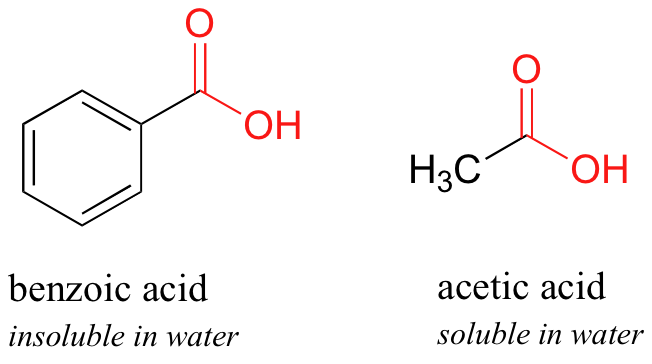
Acetic acid, withal, is quite soluble. This is piece of cake to explain using the small alcohol vs large booze argument: the hydrogen-bonding, hydrophilic effect of the carboxylic acid group is powerful enough to overcome the hydrophobic result of a single methyl group on acetic acid, but not the larger hydrophobic issue of the 6-carbon benzene group on benzoic acid.
Now, endeavor slowly calculation some aqueous sodium hydroxide to the flask containing undissolved benzoic acrid. As the solvent becomes more and more basic, the benzoic acid begins to dissolve, until it is completely in solution.
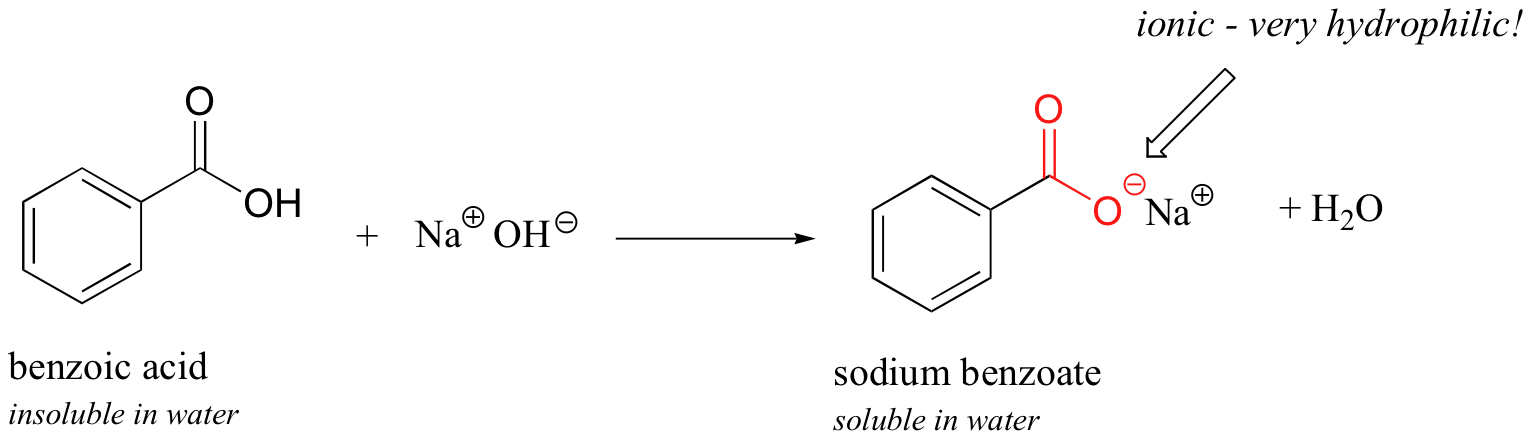
What is happening here is that the benzoic acid is existence converted to its cohabit base, benzoate. The neutral carboxylic acrid group was non hydrophilic enough to brand upwards for the hydrophobic benzene ring, but the carboxylate group, with its full negative charge, is much more hydrophilic. Now, the balance is tipped in favor of water solubility, every bit the powerfully hydrophilic anion part of the molecule drags the hydrophobic role, kicking and screaming, (if a benzene ring can kick and scream) into solution. If you want to precipitate the benzoic acrid back out of solution, you can simply add enough hydrochloric acid to neutralize the solution and reprotonate the carboxylate.
If you are taking a lab component of your organic chemistry class, yous will probably practise at least one experiment in which you will use this phenomenon to carve up an organic acid similar benzoic acid from a hydrocarbon compound similar biphenyl.
Similar arguments can be fabricated to rationalize the solubility of different organic compounds in nonpolar or slightly polar solvents. In general, the greater the content of charged and polar groups in a molecule, the less soluble it tends to be in solvents such as hexane. The ionic and very hydrophilic sodium chloride, for example, is not at all soluble in hexane solvent, while the hydrophobic biphenyl is very soluble in hexane.
Exercise
1. Vitamins tin be classified as water-soluble or fat-soluble (consider fat to exist a very non-polar, hydrophobic 'solvent'. Make up one's mind on a classification for each of the vitamins shown below.
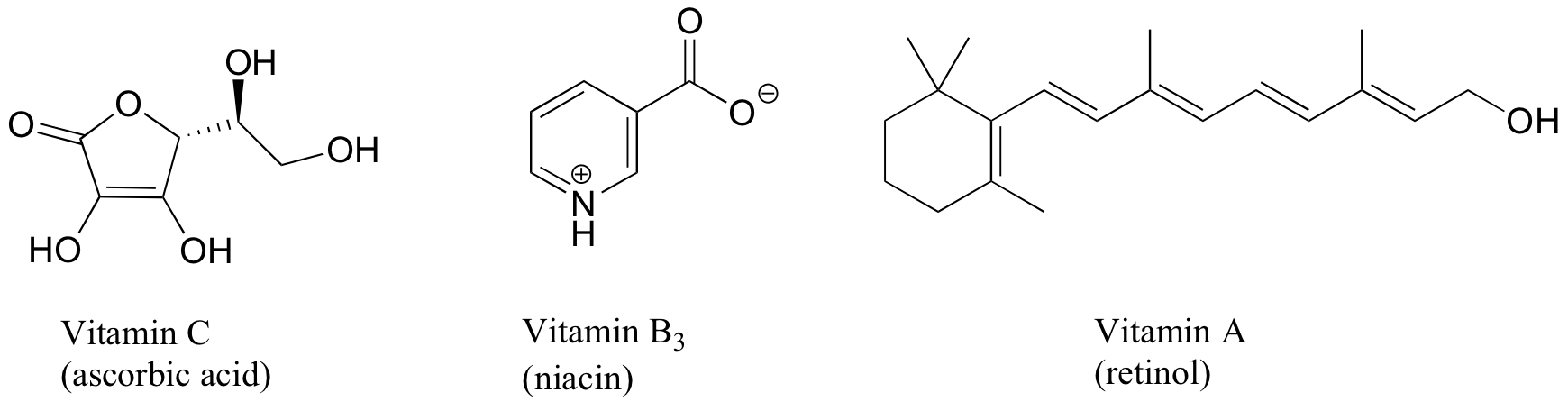
Solutions
Exercise
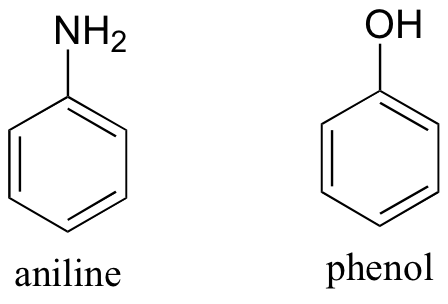
2. Both aniline and phenol are insoluble in pure water. Predict the solubility of these two compounds in 10% aqueous hydrochloric acid, and explain your reasoning. Hint – in this context, aniline is basic, phenol is not!
Solutions
Illustrations of solubility concepts: metabolic intermediates, lipid bilayer membranes, soaps and detergents
Because water is the biological solvent, most biological organic molecules, in order to maintain h2o-solubility, contain one or more charged functional groups. These are well-nigh often phosphate, ammonium or carboxylate, all of which are charged when dissolved in an aqueous solution buffered to pH 7.
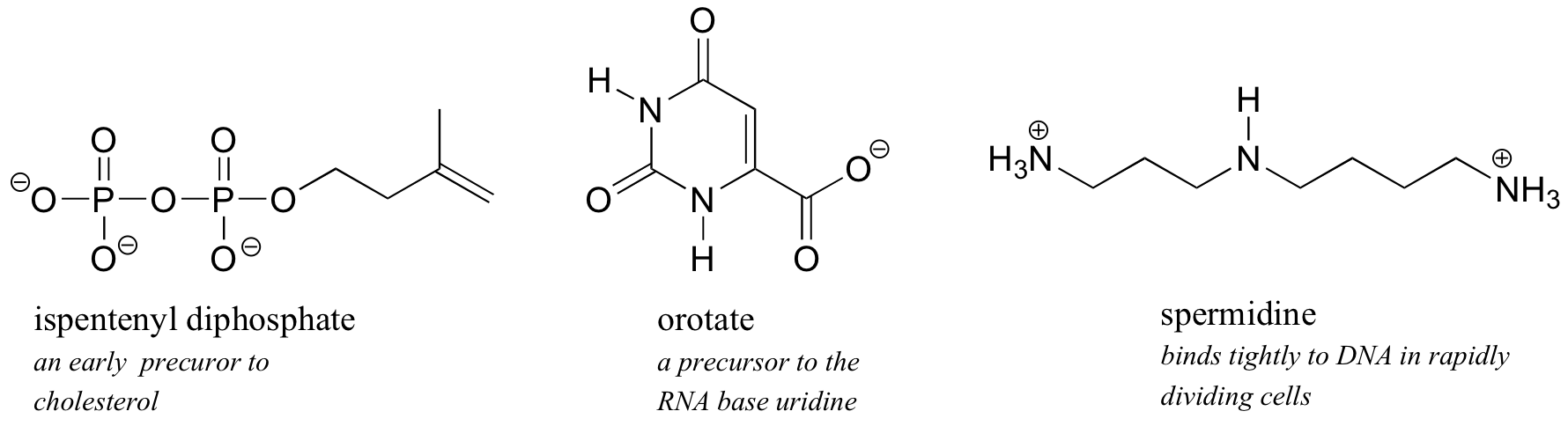
Sugars frequently lack charged groups, but as we discussed in our 'thought experiment' with glucose, they are quite water-soluble due to the presence of multiple hydroxyl groups.
Some biomolecules, in contrast, contain distinctly nonpolar, hydrophobic components. The 'lipid bilayer' membranes of cells and subcellular organelles serve to enclose volumes of water and myriad biomolecules in solution. The lipid (fat) molecules that brand up membranes are amphipathic: they take a charged, hydrophilic 'head' and a hydrophobic hydrocarbon 'tail'.
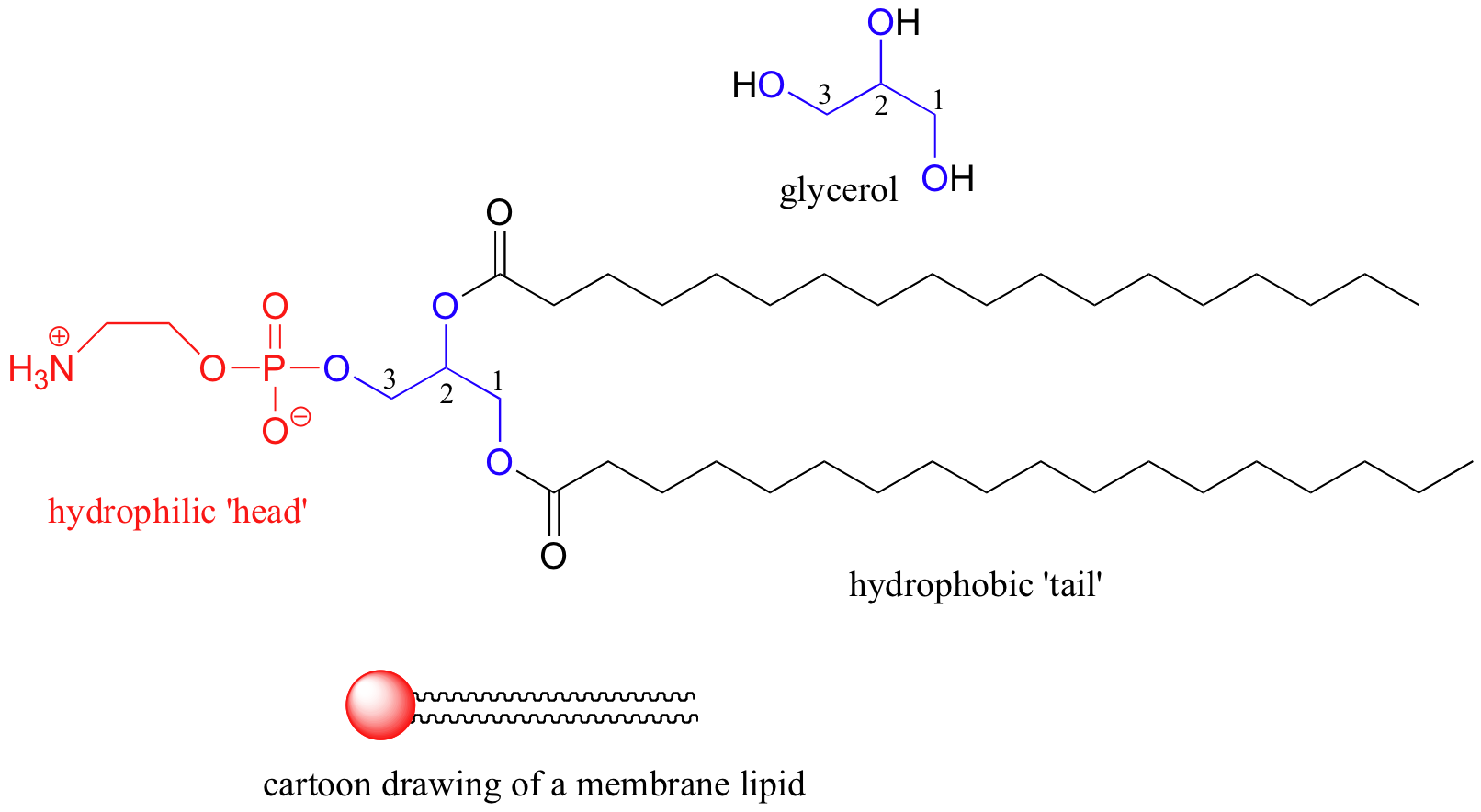
interactive 3D image of a membrane phospholipid (BioTopics)
Detect that the entire molecule is built on a 'courage' of glycerol, a elementary 3-carbon molecule with three alcohol groups. In a biological membrane structure, lipid molecules are bundled in a spherical bilayer: hydrophobic tails bespeak in and bind together by London dispersion forces, while the hydrophilic head groups course the inner and outer surfaces in contact with water.
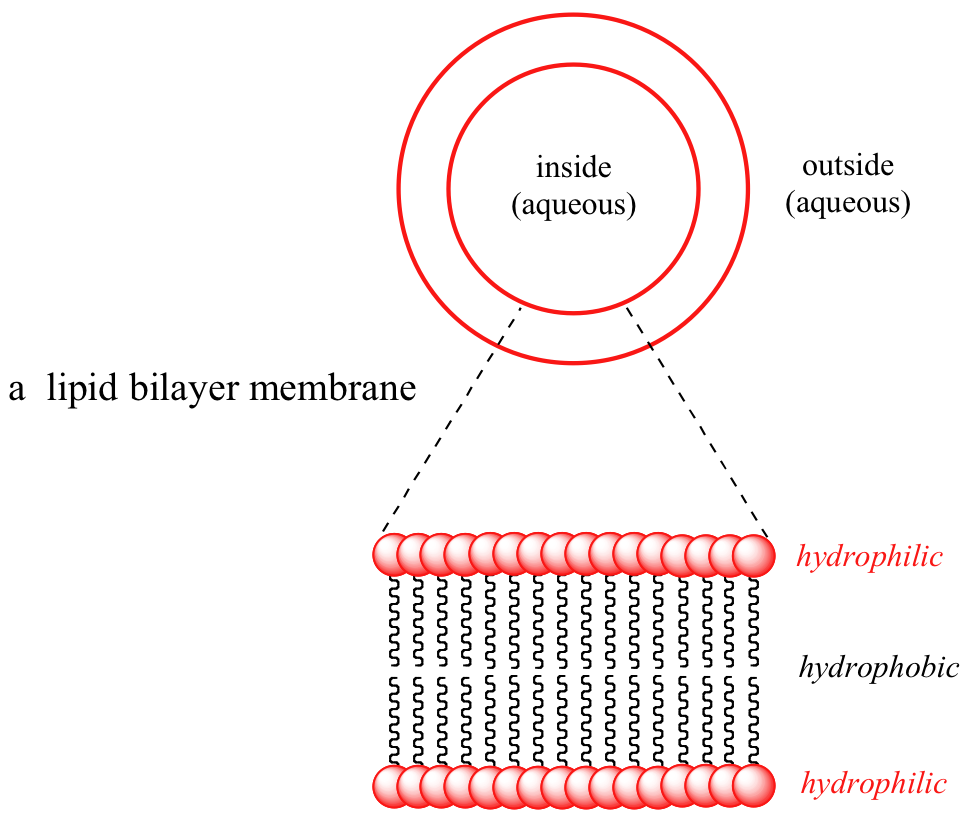
Interactive 3D Prototype of a lipid bilayer (BioTopics)
Because the interior of the bilayer is extremely hydrophobic, biomolecules (which as nosotros know are generally charged species) are not able to diffuse through the membrane– they are but not soluble in the hydrophobic interior. The transport of molecules across the membrane of a prison cell or organelle can therefore be accomplished in a controlled and specific manner by special transmembrane transport proteins, a fascinating topic that you volition learn more about if you accept a class in biochemistry.
A similar principle is the footing for the activity of soaps and detergents. Soaps are equanimous of fatty acids, which are long (typically xviii-carbon), hydrophobic hydrocarbon chains with a (charged) carboxylate group on ane stop,
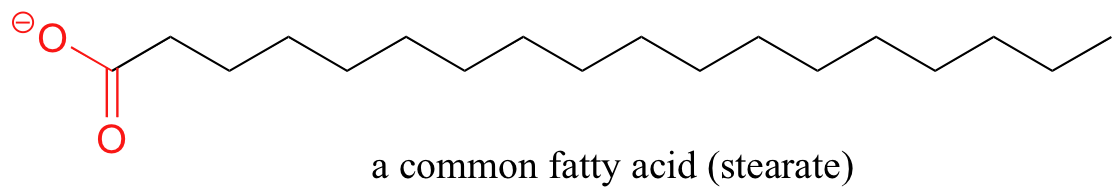
Fatty acids are derived from animate being and vegetable fats and oils. In aqueous solution, the fatty acid molecules in soaps will spontaneously grade micelles, a spherical structure that allows the hydrophobic tails to avoid contact with water and simultaneously course favorable London dispersion contacts.
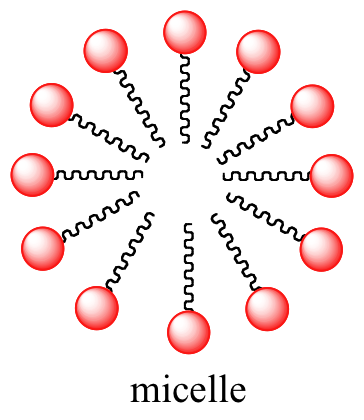
Interactive 3D images of a fatty acid soap molecule and a soap micelle (Edutopics)
Because the outside of the micelle is charged and hydrophilic, the structure as a whole is soluble in water. Micelles will form spontaneously around modest particles of oil that normally would not dissolve in h2o (like that greasy spot on your shirt from the pepperoni slice that fell off your pizza), and will acquit the particle away with information technology into solution. We volition learn more almost the chemistry of soap-making in a later chapter (section 12.4B).
Synthetic detergents are non-natural amphipathic molecules that work past the same principle as that described for soaps.
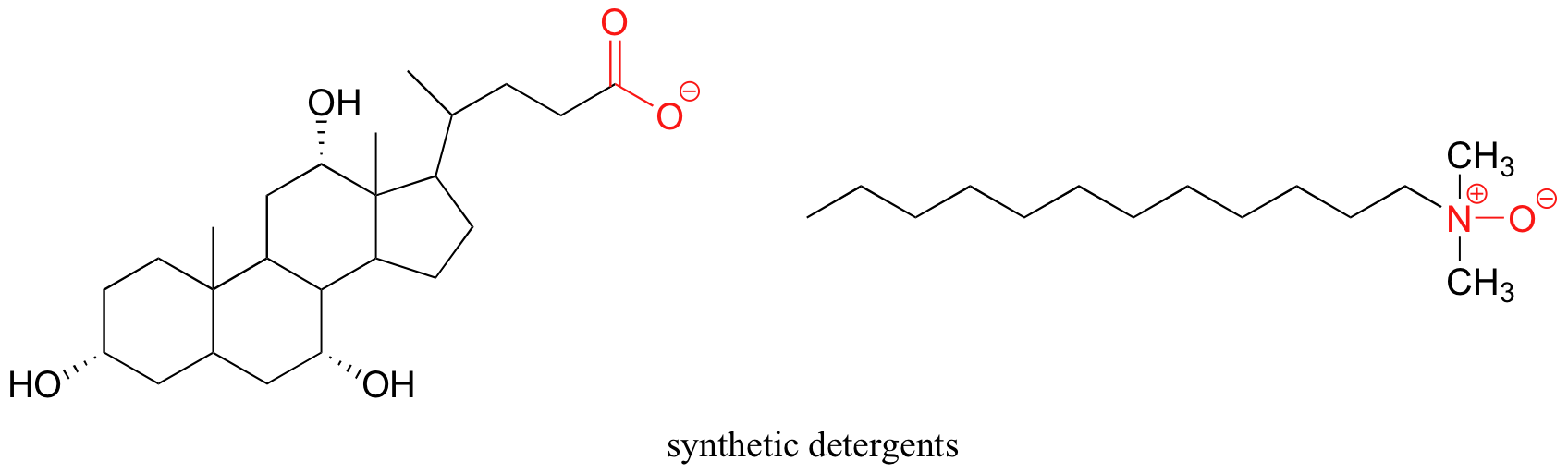
Does Hexane Have Hydrogen Bonding,
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(Wade)/02%3A_Structure_and_Properties_of_Organic_Molecules/2.12%3A_Intermolecular_Forces_and_Solubilities
Posted by: lucktope2001.blogspot.com


0 Response to "Does Hexane Have Hydrogen Bonding"
Post a Comment