Is The Ph Scale Logarithmic
The pH Scale
- Page ID
- 1291
Learning Objectives
- To ascertain the pH scale every bit a measure of acerbity of a solution
- Tell the origin and the logic of using the pH scale.
- Employ the same strategy for representing other types of quantities such as pK a, pK b, pG w.
Auto-Ionization of Water
Because of its amphoteric nature (i.eastward., acts every bit both an acid or a base), water does not always remain as \(H_2O\) molecules. In fact, two water molecules react to form hydronium and hydroxide ions:
\[ \ce{ 2 H_2O (l) \rightleftharpoons H_3O^+ (aq) + OH^{−} (aq)} \label{1}\]
This is also chosen the cocky-ionization of water. The concentration of \(H_3O^+\) and \(OH^-\) are equal in pure water because of the i:one stoichiometric ratio of Equation \(\ref{1}\). The molarity of HiiiO+ and OH- in h2o are also both \(1.0 \times ten^{-7} \,M\) at 25° C. Therefore, a constant of water (\(K_w\)) is created to prove the equilibrium condition for the self-ionization of h2o. The product of the molarity of hydronium and hydroxide ion is always \(1.0 \times ten^{-14}\) (at room temperature).
\[K_w= [H_3O^+][OH^-] = 1.0 \times x^{-14} \characterization{2}\]
Equation \(\ref{2}\) likewise applies to all aqueous solutions. However, \(K_w\) does alter at different temperatures, which affects the pH range discussed beneath.
\(H^+\) and \(H_3O^+\) is often used interchangeably to represent the hydrated proton, commonly telephone call the hydronium ion.
Equation \ref{1} can also exist written as
\[ H_2O \rightleftharpoons H^+ + OH^- \label{3}\]
Equally expected for any equilibrium, the reaction tin be shifted to the reactants or products:
- If an acid (\(H^+\)) is added to the water, the equilibrium shifts to the left and the \(OH^-\) ion concentration decreases
- If base ( \(OH^-\)) is added to water, the equilibrium shifts to left and the \(H^+\) concentration decreases.
pH and pOH
Because the constant of water, Kdue west is \(i.0 \times 10^{-fourteen}\) (at 25° C), the \(pK_w\) is xiv, the abiding of water determines the range of the pH calibration. To sympathize what the pKw is, it is important to understand first what the "p" means in pOH and pH. The addition of the "p" reflects the negative of the logarithm, \(-\log\). Therefore, the pH is the negative logarithm of the molarity of H, the pOH is the negative logarithm of the molarity of \(\ce{OH^-}\), and the \(pK_w\) is the negative logarithm of the constant of water:
\[ \brainstorm{marshal} pH &= -\log [H^+] \label{4a} \\[4pt] pOH &= -\log [OH^-] \label{4b} \\[4pt] pK_w &= -\log [K_w] \label{4c} \stop{align}\]
At room temperature,
\[K_w =1.0 \times ten^{-14} \label{4d}\]
So
\[\brainstorm{align} pK_w &=-\log [1.0 \times 10^{-fourteen}] \characterization{4e} \\[4pt] &=xiv \end{marshal}\]
Using the properties of logarithms, Equation \(\ref{4e}\) tin can exist rewritten equally
\[ten^{-pK_w}=10^{-xiv}. \label{4f}\]
The equation also shows that each increasing unit on the scale decreases by the gene of ten on the concentration of \(\ce{H^{+}}\). Combining Equations \ref{4a} - \ref{4c} and \ref{4e} results in this of import human relationship:
\[pK_w= pH + pOH = 14 \label{5b}\]
Equation \ref{5b} is right simply at room temperature since changing the temperature volition change \(K_w\).
The pH scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration past a tenfold. For case, a pH of 3 is ten times more than acidic than a pH of 4. Too, a pH of three is i hundred times more than acidic than a pH of 5. Similarly a pH of 11 is ten times more than basic than a pH of x.
Properties of the pH Scale
From the unproblematic definition of pH in Equation \ref{4a}, the following properties can be identified:
- This scale is convenient to use, because information technology converts some odd expressions such as \(1.23 \times 10^{-4}\) into a unmarried number of three.91.
- This scale covers a very big range of \(\ce{[H+]}\), from 0.1 to 10-14. When \(\ce{[H+]}\) is high, we usually exercise not use the pH value, merely simply the \(\ce{[H+]}\). For example, when \(\mathrm{[H^+] = 1.0}\), pH = 0. We seldom say the pH is 0, and that is why you consider pH = 0 such an odd expression. A pH = -0.30 is equivalent to a \(\ce{[H+]}\) of two.0 M. Negative pH values are but for academic exercises. Using the concentrations directly conveys a better sense than the pH scales.
- The pH calibration expands the division between zero and 1 in a linear scale or a compact calibration into a large scale for comparison purposes. In mathematics, you learned that there are infinite values between 0 and i, or between 0 and 0.one, or between 0 and 0.01 or between 0 and any modest value. Using a log scale certainly converts infinite small quantities into infinite large quantities.
- The non-linearity of the pH scale in terms of \(\ce{[H+]}\) is hands illustrated by looking at the corresponding values for pH between 0.1 and 0.9 equally follows:
| pH | 0 | 0.ane | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.seven | 0.8 | 0.9 |
|---|---|---|---|---|---|---|---|---|---|---|
| [H+] | ane | 0.79 | 0.63 | 0.50 | 0.40 | 0.32 | 0.25 | 0.20 | 0.16 | 0.thirteen |
- Considering the negative log of \(\ce{[H+]}\) is used in the pH scale, the pH calibration unremarkably has positive values. Furthermore, the larger the pH, the smaller the \(\ce{[H+]}\).
The Constructive Range of the pH Calibration
It is common that the pH scale is argued to range from 0-14 or perhaps 1-14, merely neither is correct. The pH range does not have an upper nor lower bound, since every bit defined above, the pH is an indication of concentration of H+. For example, at a pH of zero the hydronium ion concentration is one molar, while at pH 14 the hydroxide ion concentration is one molar. Typically the concentrations of H+ in water in most solutions fall between a range of 1 Thousand (pH=0) and 10-14 M (pH=14). Hence a range of 0 to 14 provides sensible (but not absolute) "bookends" for the scale. 1 can go somewhat below zippo and somewhat higher up 14 in water, considering the concentrations of hydronium ions or hydroxide ions can exceed one molar. Figure \(\PageIndex{one}\) depicts the pH scale with common solutions and where they are on the scale.
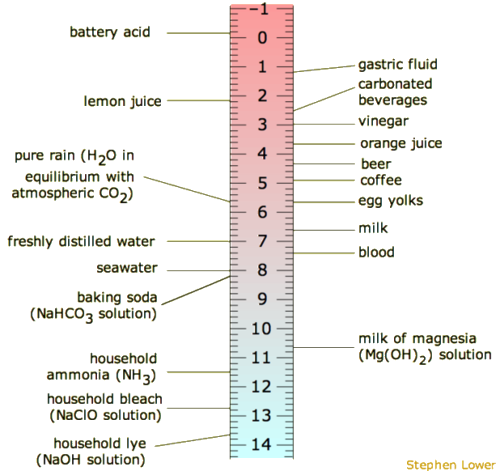
Quick Interpretation
- If pH >7, the solution is bones. The pOH should be looked in the perspective of OH- instead of H+. Whenever the value of pOH is less than seven, and so it is considered basic. And therefore in that location are more OH- than H+ in the solution.
- At pH 7, the substance or solution is at neutral and means that the concentration of H+ and OH- ion is the same.
- If pH < seven, the solution is acidic. There are more H+ than OH- in an acidic solution.
- The pH scale does not have an upper nor lower bound.
Example \(\PageIndex{1}\)
If the concentration of \(NaOH\) in a solution is \(two.5 \times x^{-4}\; G\), what is the concentration of \(H_3O^+\)?
Solution
We can presume room temperature, and so
\[1.0 \times x^{-14} = [H_3O^+][OH^-] \nonumber\]
to notice the concentration of HthreeO+, solve for the [HiiiO+].
\[\dfrac{i.0 \times 10^{-14}}{[OH^-]} = [H_3O^+]\]
\[\dfrac{1.0 \times 10^{-14}}{2.5 \times ten^{-four}} = [H_3O^+] = 4.0 \times ten^{-11}\; M\]
Example \(\PageIndex{2}\)
- Find the pH of a solution of 0.002 M of HCl.
- Discover the pH of a solution of 0.00005 M NaOH.
Solution
- The equation for pH is -log [H+]
\[[H^+]= ii.0 \times 10^{-three}\; M \nonumber\]
\[pH = -\log [2.0 \times 10^{-3}] = ii.70 \nonumber\]
- The equation for pOH is -log [OH-]
\[ [OH^-]= five.0 \times x^{-v}\; Grand \nonumber\]
\[pOH = -\log [5.0 \times 10^{-5}] = 4.30 \nonumber\]
\[pK_w = pH + pOH \nonumber\]
and
\[pH = pK_w - pOH \nonumber\]
and so
\[pH = fourteen - 4.30 = 9.lxx \nonumber\]
Instance \(\PageIndex{iii}\): Soil
If moist soil has a pH of 7.84, what is the H+ concentration of the soil solution?
Solution
\[pH = -\log [H^+] \nonumber\]
\[7.84 = -\log [H^+] \nonumber\]
\[[H^+] = 1.45 \times 10^{-8} M \nonumber\]
Hint
Place -7.84 in your calculator and have the antilog (often inverse log or 1010) = i.45 x 10-8Thousand
Proper Definition of pH
The pH scale was originally introduced by the Danish biochemist S.P.Fifty. Sørenson in 1909 using the symbol pH. The letter p is derived from the German word potenz meaning power or exponent of, in this case, 10. In 1909, Due south.P.L. Sørenson published a paper in Biochem Z in which he discussed the consequence of H+ ions on the activeness of enzymes. In the newspaper, he invented the term pH (purported to mean pondus hydrogenii in Latin) to describe this effect and defined it equally the \(-\log[H^+]\). In 1924, Sørenson realized that the pH of a solution is a function of the "activity" of the H+ ion and not the concentration. Thus, he published a second paper on the subject. A amend definition would exist
\[pH = -\log\,a\{\ce{H^{+}}\}\]
where \(a\{H^+\}\) denotes the activity (an constructive concentration) of the H+ ions. The activeness of an ion is a part of many variables of which concentration is 1.
- Concentration is abbreviated by using square brackets, east.g., \([H_3O^+]\) is the concentration of hydronium ion in solution.
- Activity is abbreviated by using "a" with curly brackets, eastward.thousand., \(a\{H_3O^+\}\) is the activity of hydronium ions in solution
Because of the difficulty in accurately measuring the activity of the \(\ce{H^{+}}\) ion for most solutions the International Union of Pure and Practical Chemistry (IUPAC) and the National Agency of Standards (NBS) has defined pH as the reading on a pH meter that has been standardized against standard buffers. The following equation is used to calculate the pH of all solutions:
\[\begin{marshal} pH &= \dfrac{F(East-E_{standard})}{RT\;\ln 10} + pH_{standard} \label{6a} \\[4pt] &= \dfrac{5039.879 (East-E_{standard})}{T} + pH_{standard} \characterization{6b} \end{align}\]
with
- \(R\) is the ideal gas abiding,
- \(F\) is the Faraday's constant, and
- \(T\) is absolute temperature (in Chiliad)
The action of the H+ ion is determined as accurately as possible for the standard solutions used. The identity of these solutions vary from one authority to another, but all give the same values of pH to ± 0.005 pH unit. The historical definition of pH is correct for those solutions that are so dilute and so pure the H+ ions are not influenced by anything but the solvent molecules (usually water).
When measuring pH, [H+] is in units of moles of H+ per liter of solution. This is a reasonably accurate definition at low concentrations (the dilute limit) of H+. At very high concentrations (10 M hydrochloric acrid or sodium hydroxide, for example,) a meaning fraction of the ions will be associated into neutral pairs such as H + Cl–, thus reducing the concentration of "available" ions to a smaller value which we will call the effective concentration. It is the effective concentration of H + and OH– that determines the pH and pOH. The pH scale as shown above is called sometimes "concentration pH scale" as opposed to the "thermodynamic pH scale". The main difference between both scales is that in thermodynamic pH scale 1 is interested not in H+concentration, but in H+activity. What a person measures in the solution is just activity, not the concentration. Thus it is thermodynamic pH calibration that describes real solutions, not the concentration one.
For solutions in which ion concentrations don't exceed 0.1 M, the formulas pH = –log [H + ] and pOH = –log[OH–] are more often than not reliable, but don't wait a 10.0 Grand solution of a strong acrid to have a pH of exactly –1.00! However, this definition is only an approximation (albeit very good under most situations) of the proper definition of pH, which depends on the activity of the hydrogen ion:
\[pH= -\log a\{H^+\} \approx -\log [H^+] \label{vii}\]
The activity is a measure of the "constructive concentration" of a substance, is oft related to the true concentration via an activity coefficient, \(\gamma\):
\[a{H^+}=\gamma [H^+] \characterization{8}\]
Calculating the activity coefficient requires detailed theories of how charged species interact in solution at high concentrations (eastward.grand., the Debye-Hückel Theory). In nigh solutions the pH differs from the -log[H+ ] in the kickoff decimal indicate. The following table gives experimentally determined pH values for a series of HCl solutions of increasing concentration at 25 °C.
| Tooth Concentration of \(HCl\) | pH defined as Concentration | Experimentally Determined pH | Relative Departure |
|---|---|---|---|
| 0.00050 | 3.30 | iii.31 | 0.three% |
| 0.0100 | 2 | ii.04 | 1.9% |
| 0.100 | 1 | 1.10 | ix% |
| 0.40 | 0.39 | 0.52 | 25% |
| seven.6 | -0.88 | -1.85 | 52% |
While the pH scale formally measures the activeness of hydrogen ions in a substance or solution, it is typically approximated equally the concentration of hydrogen ions; this approximation is applicative but nether low concentrations.
Living Systems
Molecules that make upward or are produced past living organisms commonly role within a narrow pH range (near neutral) and a narrow temperature range (body temperature). Many biological solutions, such as blood, have a pH near neutral. pH influences the structure and the function of many enzymes (poly peptide catalysts) in living systems. Many of these enzymes accept narrow ranges of pH activity. Cellular pH is so important that death may occur within hours if a person becomes acidotic (having increased acerbity in the claret). Equally one tin run into pH is critical to life, biochemistry, and important chemical reactions. Common examples of how pH plays a very of import role in our daily lives are given below:
- Water in pond pool is maintained past checking its pH. Acidic or basic chemicals can be added if the water becomes as well acidic or too bones.
- Whenever we get a heartburn, more acid build up in the stomach and causes hurting. We needs to take antacid tablets (a base of operations) to neutralize excess acid in the breadbasket.
- The pH of blood is slightly basic. A fluctuation in the pH of the blood tin can crusade in serious impairment to vital organs in the body.
- Certain diseases are diagnosed but by checking the pH of blood and urine.
- Certain crops thrive amend at certain pH range.
- Enzymes activate at a certain pH in our body.
| Compartment | pH |
|---|---|
| Gastric Acrid | 1 |
| Lysosomes | 4.5 |
| Granules of Chromaffin Cells | 5.v |
| Human Peel | five.v |
| Urine | six |
| Neutral H2O at 37 °C | half dozen.81 |
| Cytosol | 7.2 |
| Cerebrospinal Fluid | 7.3 |
| Blood | seven.43-seven.45 |
| Mitochondrial Matrix | 7.v |
| Pancreas Secretions | 8.one |
Problems
- In a solution of \(two.4 \times 10^{-three} M\) of Hi, discover the concentration of \(OH^-\).
- Decide the pH of a solution that is 0.0035 M HCl.
- Determine the [HthreeO+] of a solution with a pH = v.65
- If the pOH of NH3, ammonia, in water is 4.74. What is the pH?
- Pepsin, a digestive enzyme in our breadbasket, has a pH of 1.5. Find the concentration of OH- in the stomach.
Solutions
- We use the dissociation of water equation to observe [OH-].
Yardw = [HthreeO+][OH-] = i.0 X 10-xiv
Solve for [OH-]
[OH-] = (ane.0 X x-xiv)/ [H3O+]
Plug in the molarity of HI and solve for OH-.
[OH-] = (1.0 10 10-14)/ [2.four Ten ten-3] = 4.17 Ten 10-12 M. - pH = -log[HthreeO+]
Plug the molarity of the HCl in and solve for pH.
pH = -log[0.0035] = 2.46 - pH = -log[HthreeO+]
Plug in the pH and solve for [H3O+]
5.65 = -log[H3O+]
Motility the negative sign to the pH. -5.65 = log[H3O+]
10-five.65=xlog [H3O+] = 2.24 10 10-half-dozen M - pH + pOH = fourteen
Solve for pH.
14 - pOH = pH
fourteen - 4.74 = pH = 9.26 - In that location are several ways to do this problem.
Answer 1.
pH + pOH = 14
Solve for pOH.
pOH = 14 - pH
pOH = 14 - 1.v = 12.5
When the pOH is solved, solve for the concentration by using log.
pOH = -log[OH-]
12.5 = -log[OH-]
-12.5 = log[OH-]
10-12.five = tenlog [OH-] = iii.16 X x-thirteen M.Answer two.
pH = -log[H+]
Plug in the pH and solve for the molarity of H+ of pepsin.
one.5 = -log[H+]
-1.5 = log[H+]
10-ane.5 = xl og [H+] = [H+]= 0.032
Use the concentration of H+ to solve for the concentration of OH-.
[H+][OH-] = 1.0 X 10-14
Plug in the [H+] and solve for [OH-].
[OH-] = (one.0 X 10-14)/[HiiiO+]
[OH-] = (1.0 Ten 10-14)/(0.032) = iii.125 Ten 10-14 G
References
- Petrucci, et al. "Self-Ionization of Water and the pH Scale." General Chemistry: Principles & Modern Applications. 7th ed. Upper Saddle River: Pearson Prentice Hall, 2007. 669-71.
- Segel, Irwin H. "Acid and Base of operations." Biochemical Calculations. 2d ed. Wiley: BK Volume, 1976. 12.
- Christopher G. McCarty and Ed Vitz, Journal of Chemical Instruction, 83(5), 752 (2006)
Is The Ph Scale Logarithmic,
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Acids_and_Bases/Acids_and_Bases_in_Aqueous_Solutions/The_pH_Scale
Posted by: lucktope2001.blogspot.com


0 Response to "Is The Ph Scale Logarithmic"
Post a Comment